Greensburg salem school district
GREENSBURG SALEM SCHOOL DISTRICT 2013 – 2014 ‘GOLDEN LION’ BANDS MEDICAL INFORMATION FORM Please print/type all information, sign and notarize on rear of form, and return by 8/7 Note: Only NEW members need to have this form notarized! NAME: _____________________________________ SECTION: ________________________________ ADDRESS: _________________________________________
 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
16 Determining Dissociation Constants
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
16 Determining Dissociation Constants 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
There are a number of ways that dissociation constants can be calculated. One technique that you may be the most familiar with is the use of a pH meter to determine the dissociation constant of an acid or base (Ka or Kb) in water by conducting a titration. Another method uses a spectrophotometer to determine the concentrations of ionized and unionized forms based on their absorbance spectra. A third way to determine dissociation constants is by measuring the conductivity of a solution at varying concentrations. In this exploration, you will be utilizing this third method.
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
There are a number of ways that dissociation constants can be calculated. One technique that you may be the most familiar with is the use of a pH meter to determine the dissociation constant of an acid or base (Ka or Kb) in water by conducting a titration. Another method uses a spectrophotometer to determine the concentrations of ionized and unionized forms based on their absorbance spectra. A third way to determine dissociation constants is by measuring the conductivity of a solution at varying concentrations. In this exploration, you will be utilizing this third method. 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Materials
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Materials
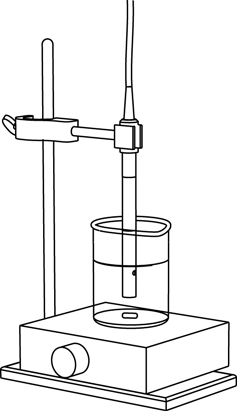 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Xplorer GLX Setup
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Xplorer GLX Setup 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
e In the Calibration Screen, the Calibration Type should be listed as 1 Point Slope.
If it is not, use the Arrow keys to highlight the Calibration Type. Press
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
e In the Calibration Screen, the Calibration Type should be listed as 1 Point Slope.
If it is not, use the Arrow keys to highlight the Calibration Type. Press  1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Watch the Digits display and wait for the conductivity to stabilize. Record this value in your table.
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Watch the Digits display and wait for the conductivity to stabilize. Record this value in your table. 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Data Table
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Data Table 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Calculate the values for c/ and 1// and enter them in the following table (c = inital molar concentation).
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
Calculate the values for c/ and 1// and enter them in the following table (c = inital molar concentation). 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
and select 1/ac as the data source for the x axis.
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
and select 1/ac as the data source for the x axis. 1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y
1 6 D E T E R M I N I N G D I S S O C I A T I O N C O N S T A N T S — L A B A C T I V I T Y