Do you want to buy antibiotics online without prescription? https://buyantibiotics24h.net/ - This is pharmacy online for you!
Untitled
Preventive Effects of
Rosiglitazone on Restenosis
after Coronary Stenting in
Patients with Type 2 Diabetes
Donghoon Choi, MD, PhD
Cardiology Division
Yonsei University College of Medicine,
Background
1. Cardiovascular disease is one of the important leading
cause of deaths in Type 2 diabetic patients.
2. As a result of dramatic increase in implantation
numbers, in-stent restenosis has been significant clinical and socio-economic problems.
3. The in-stent restenosis rate after coronary stenting has
reached up to 45-50 % in type 2 DM patients comparing to 15-25% in non-diabetic patients.
4. The most effective treatment modality for in-stent
Pathogenesis of Restenosis
Growth factors & cytokines
Receptor activation
Smooth muscle cell
Cell proliferation
Extracellular matrix
Migration
Synthesis & secretion
Approaches for Restenosis
Prevention
Inflammation
Migration
Proliferation
Reduce injury 1. Enhance biocompatibility
2. Anti-inflammatory
Antimigratory
Antiproliferative
Promote healing
& reendotheliali-
Atherogenic Effects of PPAR γ
Ligands
in the Vasculature
Monocytes
Endothelial Cells
↓
Atherosclerosis
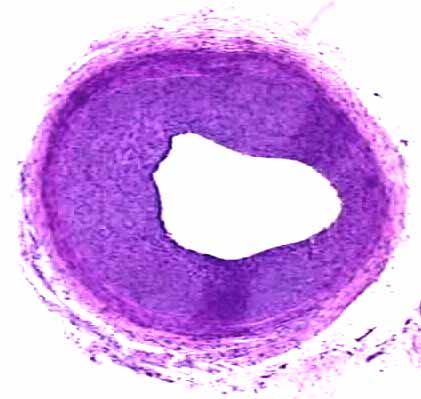
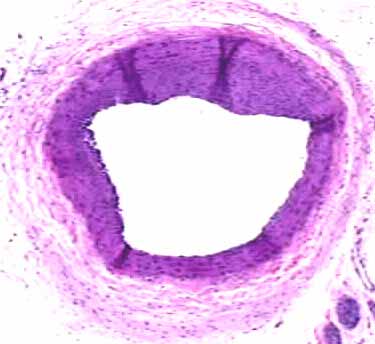
Male OLETF rat, Balloon injury at 16 weeks
and pioglitazone for 3weeks
Intima area
Intima/media ratio
Pioglitazone
Pioglitazone
TZDs: effects on carotid arterial intimal and medial co
Troglitazone 400 mg/day
IMT (mm) ∆
Japanese subjects with type 2 diabetes*
P < 0.001 vs. control
Minamikawa J, et al. J Clin Endocrinol Metab 1998; 83:1818–1820.
Study Purpose
• To investigate the preventive effect of PPAR-
γ agonist, rosiglitazone on restenosis after coronary stenting in type II DM patients.
=> 6 month follow-up angiographic binary
Subjects (I)
- Type II DM patients undergoing coronary
stenting at YUMC (Nov. 2001 ~ Dec. 2002)
- LVEF < 40% or evidence of CHF- GOT/GPT > 2 x upper limit of normal range- Cr > 2.0 mg/DL - Previous CABG- Primary PTCA
Subjects (II)
Study design and Method
• Anthropometry, Serologic lab : initial and 6 month
• Rosiglotazone : at least 8mg before angiography,
• Control Blood Sugar : continue individual
conventional therapy (sulfonylurea, biguanide,
Baseline Characteristics
Rosiglitazone
No. (male/female)
45 (34/11)
38 (24/14)
Age (years)
59.9 ±
9.3
60.9 ±
9.3
DM duration (years)
7.2 ±
3.8
7.5 ±
4.9
BMI (kg/cm2)
24.8 ±
3.35
24.9 ±
2.96
Fasting glucose (mg/dL)
150.3 ±
28.4
160.3 ±
34.4
HbA1c (%)
7.72 ±
1.13
7.79 ±
1.30
Fasting insulin ( µ
U/mL)
4.97 ±
2.51
5.60 ±
2.70
Total cholesterol (mg/dL)
191.1 ±
48.9
190.5 ±
37.6
HDL-cholesterol (mg/dL)
41.1 ±
10.9
38.9 ±
11.0
Triglyceride (mg/dL)
159.5 ±
55.1
167.7 ±
60.8
Free fatty acid ( µ
mol/L)
580.3 ±
101.7
669.2 ±
127.4
hsCRP (mg/L)
2.01 ±
1.33
2.92 ±
1.98
Medications
Rosiglitazone
Treatments: No. (%)
HMG-CoA reductase
37 (88.1)
31 (81.6)
inhibitor
ACE inhibitors
30 (71.4)
28 (73.7)
Antiplatelet agents
38 (90.5)
34 (89.5)
Sulfonylureas
26 (61.9)
25 (65.8)
Biguanides
22 (52.3)
21 (55.3)
α
-glucosidase inhibitor
15 (35.7)
10 (26.3)
Baseline Angiographic
Characteristics
Rosiglitazone
Stented coronary vessels
Left main
Reference diameter (mm)
3.15 ±
0.49
3.16 ±
0.49
Minimum lumen diameter
0.65 ±
0.41
0.83 ±
0.57
Diameter stenosis (%)
79.4 ±
12.8
74.4 ±
15.8
Lesion length (mm)
16.48 ±
5.16
19.02 ±
6.09
<0.05
Post-stenting Angiographic Data
Rosiglitazone
Stent diameter (mm)
3.24 ±
0.42
3.29 ±
0.41
Stent length (mm)
18.40 ±
4.75
20.28 ±
5.73
Post-stenting
3.10 ±
0.43
3.13 ±
0.48
Diameter stenosis (%)
2.49 ±
4.26
2.25 ±
4.44
Acute gain (mm)
2.45 ±
0.57
2.30 ±
0.53
Follow-up Biochemical
Characteristics
Rosiglitazone
Baseline FU
Baseline FU
Fasting glucose (mmol/l)
8.34 ±
1.58 6.87 ±
1.52
8.90 ±
1.91 7.35 ±
1.89
HbA1c (%)
7.72 ±
1.13 7.23 ±
0.93
7.79 ±
1.30 7.17 ±
0.98
Fasting insulin (pmol/l)
35.7 ±
18.0 34.2 ±
18.9
40.2 ±
19.4 34.5 ±
19.7
HDL-cholesterol (mmol/l)
1.06 ±
0.28 1.14 ±
0.27
1.01 ±
0.28 1.12 ±
0.21
Triglyceride (mmol/l)
1.80 ±
0.62 1.43 ±
0.69
1.89 ±
0.69 1.34 ±
0.44
Free fatty acid ( µ
mol/L)
580.3 ±
101.7 548 ±
95.6
669.2 ±
127.4 492.0 ±
101.4
hsCRP (mg/L)
2.01 ±
1.33 1.79 ±
1.22
2.92 ±
1.98 0.62 ±
0.44
Follow-Up Angiographic Data
Rosiglitazone
1.91 ±
1.05
2.49 ±
0.88
Diameter Stenosis (%)
40.60 ±
31.90
23.00 ±
23.40
Lumen loss (mm)
1.20 ±
0.97
0.65 ±
0.73
Loss index
0.49 ±
0.42
0.29 ±
0.31
Restenosis rate (%)
Clinical Follow-Up Data
Target lesion
revascularization
The Effects of Rosiglitazone
on VSMC migration
at 48 weeks (mm)
Change in m
Baseline 0.815
Progression rate
– 0.012*
IMT = intima-media thicknessPatients with clinically stable coronary artery disease without diabetes
RSG dose 4 mg/day for initial 8 weeks; 8 mg/day for remaining 40 weeks
*P = 0.03 vs. PBO
Sidhu JS, et al. Arterioscler Thromb Vasc Biol 2004; 24:930–934.
Conclusion
• In this study, rosiglitazone has dramatically
reduced restenosis rate of CAOD pateintswith coronary stenting in Type 2 diabetes.
• In type 2 diabetes patients with CAOD, using
PPAR-γ agonist, not only for glucose lowering and insulin sensitizing effect, but also for anti-inflammatory effect, has to be strongly considered.
Source: http://www.summitmd.com/pdf/pdf/050923_lec7.pdf
Erbium Laser Light to Deep Skin Resurfacing Pre and Post Treatment Information The information provided on this form is applicable for light to deep ER:YAG (Erbium) laser resurfacing treatments. Lighter treatments wil produce less downtime and fewer side-effects, whereas clients receiving deeper peels should expect longer healing times and increased risk of side-effects. General Informat
CONFIDENTIAL MEDICAL INFORMATION Patient’s Name:___________________________________ DOB: ________________ Home Phone: ( ) __________________ Street Address:__________________________________________________________ Work Phone: ( ) __________________City:____________________________________ State:____________ ZIP:____________ E-Mail ___________________________Social Security Number:______
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
R |
S |
T |
U |
V |
W |
X |
Y |
Z |
0-9 |
 Preventive Effects of
Preventive Effects of  Background
Background
 Pathogenesis of Restenosis
Pathogenesis of Restenosis Approaches for Restenosis
Approaches for Restenosis  Atherogenic Effects of PPARγ Ligands
Atherogenic Effects of PPARγ Ligands
 Male OLETF rat, Balloon injury at 16 weeks
Male OLETF rat, Balloon injury at 16 weeks  TZDs: effects on carotid arterial intimal and medial co
Troglitazone 400 mg/day
TZDs: effects on carotid arterial intimal and medial co
Troglitazone 400 mg/day Study Purpose
Study Purpose Subjects (I)
Subjects (I) Subjects (II)
Subjects (II) Study design and Method
Study design and Method Baseline Characteristics
Baseline Characteristics Medications
Medications Baseline Angiographic
Baseline Angiographic  Post-stenting Angiographic Data
Post-stenting Angiographic Data Follow-up Biochemical
Follow-up Biochemical  Follow-Up Angiographic Data
Follow-Up Angiographic Data Clinical Follow-Up Data
Clinical Follow-Up Data The Effects of Rosiglitazone
The Effects of Rosiglitazone  at 48 weeks (mm)
at 48 weeks (mm) Conclusion
Conclusion