Microsoft word - bois de la cambre.doc
CCN VOGELZANG CBN CCN VOGELZANG CBN VOGELZANG VOGELZANG COMPTE BANCAIRE - 210-0062146-21 C.C.N. VOGELZANG C.B.N. - BANKREKENING : Réaménagement du Bois de la Cambre – Enquête publique Dans le cadre de l’enquête publique organisée suite à la demande pour l’obtention du permis d’urbanisme et d’environnement pour le réaménagement du Bois de la Cambre, la CCN Vo
 The synergistic activity of triclosan and cipro£oxacin on bio¢lms ofSalmonella Typhimurium
Mina Tabak1,2, Keren Scher1, Michael L. Chikindas2 & Sima Yaron1
1Technion-Israel Institute of Technology, Faculty of Biotechnology and Food Engineering, Haifa, Israel; and 2Department of Food Science, Cook College,Rutgers, The State University of New Jersey, New Brunswick, NJ, USA
Israel Institute of Technology, Faculty of
Triclosan is a biocide whose wide use has raised a debate about the potential benefits
Biotechnology and Food Engineering, Haifa32000, Israel. Tel.: 1972 4 829 2940; fax:
vs. hazards of the incorporation of antimicrobials in consumer products. The
purpose of the present study was to determine whether exposure of biofilms of
Salmonella enterica serovar Typhimurium to triclosan influences the tolerance of thebacteria towards antibiotics such as ciprofloxacin and vice versa. A synergistic
Received 15 April 2009; accepted 8 September
antibiofilm activity was observed when the biofilms were treated with triclosan before
or together with ciprofloxacin, and an additive activity was observed with planktonic
Final version published online 16 October 2009.
The synergistic activity of triclosan and cipro£oxacin on bio¢lms ofSalmonella Typhimurium
Mina Tabak1,2, Keren Scher1, Michael L. Chikindas2 & Sima Yaron1
1Technion-Israel Institute of Technology, Faculty of Biotechnology and Food Engineering, Haifa, Israel; and 2Department of Food Science, Cook College,Rutgers, The State University of New Jersey, New Brunswick, NJ, USA
Israel Institute of Technology, Faculty of
Triclosan is a biocide whose wide use has raised a debate about the potential benefits
Biotechnology and Food Engineering, Haifa32000, Israel. Tel.: 1972 4 829 2940; fax:
vs. hazards of the incorporation of antimicrobials in consumer products. The
purpose of the present study was to determine whether exposure of biofilms of
Salmonella enterica serovar Typhimurium to triclosan influences the tolerance of thebacteria towards antibiotics such as ciprofloxacin and vice versa. A synergistic
Received 15 April 2009; accepted 8 September
antibiofilm activity was observed when the biofilms were treated with triclosan before
or together with ciprofloxacin, and an additive activity was observed with planktonic
Final version published online 16 October 2009.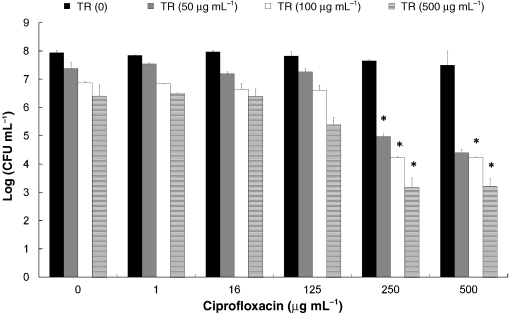 The effect of triclosan and ciprofloxacin in the biofilm
cell counts was obtained in the biofilm after 1 h of incuba-tion at 37 1C with the highest concentration used
To assess possible synergistic activity, the combination indexes
(1000 mg mLÀ1). The MIC of triclosan for planktonic cells
(CI) were calculated according to the equation: CI = (D)T/
was 0.5 mg mLÀ1. In biofilms, there was only a 1 log reduc-
(Dx)T1(D)C/(Dx)C, where (D)T and (D)C are doses of triclo-
tion even with 1000 mg mLÀ1 triclosan at 25 1C as was
san and ciprofloxacin in combination, and (Dx)T and (Dx)C
described (Tabak et al., 2007), but exposure to 500 and
are doses of triclosan and ciprofloxacin that produce x% effect
1000 mg mLÀ1 triclosan at 37 1C resulted in 1.6 and 3 log
when used alone. CI o 1, = 1, and 4 1 indicate synergism,
reduction, respectively. Because treatments with triclosan at
additive effect and antagonism, respectively. The simplest
37 1C were found to be more effective in killing the bacteria,
definition for additive effect indicates that the effect of the
we continued the experiments at 37 1C. When biofilms were
two compounds together is greater than the effect of
treated with triclosan followed by ciprofloxacin at concen-
each alone, and synergism occurs when the combined effect
trations of 50–500 mg mLÀ1 triclosan and 250–500 mg mLÀ1
exceeds that predicted by the sum of the individual actions of
ciprofloxacin, a 4–5 log reduction was observed. This reduc-
the compounds (Chou, 2006). For each set of experiments, we
tion in viability was much larger than the sum of
calculated the lowest concentrations that resulted in 90%, 99%
both compounds treated alone, indicating a possible synergy
and 99.9% reduction. Ciprofloxacin alone at a concentra-
(Fig. 1). For example, treatment with 500 mg mLÀ1 triclosan
tion as high as 1000 mg mLÀ1 resulted in approximately 80%
or ciprofloxacin reduced the CFU by 1.6 and 0.5 log,
reduction in the biofilm; thus, (Dx)C was displayed in all
respectively. However, the sequential treatment with the
calculations as 1000 mg mLÀ1, an assumption that does not
same concentration of triclosan followed by ciproflo-
affect the conclusions about synergy when the calculated
xacin resulted in a 4.8 log reduction of the viable cell count.
The effect of triclosan and ciprofloxacin in the biofilm
cell counts was obtained in the biofilm after 1 h of incuba-tion at 37 1C with the highest concentration used
To assess possible synergistic activity, the combination indexes
(1000 mg mLÀ1). The MIC of triclosan for planktonic cells
(CI) were calculated according to the equation: CI = (D)T/
was 0.5 mg mLÀ1. In biofilms, there was only a 1 log reduc-
(Dx)T1(D)C/(Dx)C, where (D)T and (D)C are doses of triclo-
tion even with 1000 mg mLÀ1 triclosan at 25 1C as was
san and ciprofloxacin in combination, and (Dx)T and (Dx)C
described (Tabak et al., 2007), but exposure to 500 and
are doses of triclosan and ciprofloxacin that produce x% effect
1000 mg mLÀ1 triclosan at 37 1C resulted in 1.6 and 3 log
when used alone. CI o 1, = 1, and 4 1 indicate synergism,
reduction, respectively. Because treatments with triclosan at
additive effect and antagonism, respectively. The simplest
37 1C were found to be more effective in killing the bacteria,
definition for additive effect indicates that the effect of the
we continued the experiments at 37 1C. When biofilms were
two compounds together is greater than the effect of
treated with triclosan followed by ciprofloxacin at concen-
each alone, and synergism occurs when the combined effect
trations of 50–500 mg mLÀ1 triclosan and 250–500 mg mLÀ1
exceeds that predicted by the sum of the individual actions of
ciprofloxacin, a 4–5 log reduction was observed. This reduc-
the compounds (Chou, 2006). For each set of experiments, we
tion in viability was much larger than the sum of
calculated the lowest concentrations that resulted in 90%, 99%
both compounds treated alone, indicating a possible synergy
and 99.9% reduction. Ciprofloxacin alone at a concentra-
(Fig. 1). For example, treatment with 500 mg mLÀ1 triclosan
tion as high as 1000 mg mLÀ1 resulted in approximately 80%
or ciprofloxacin reduced the CFU by 1.6 and 0.5 log,
reduction in the biofilm; thus, (Dx)C was displayed in all
respectively. However, the sequential treatment with the
calculations as 1000 mg mLÀ1, an assumption that does not
same concentration of triclosan followed by ciproflo-
affect the conclusions about synergy when the calculated
xacin resulted in a 4.8 log reduction of the viable cell count.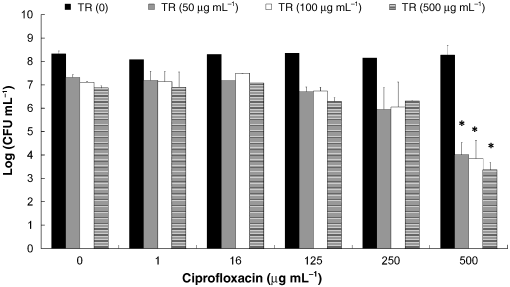
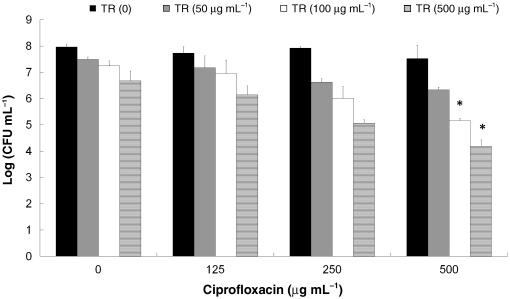 Fig. 2. The effect of treatments of biofilms ofSalmonella Typhimurium with triclosan (TR)combined with ciprofloxacin on bacterial viability.
Fig. 2. The effect of treatments of biofilms ofSalmonella Typhimurium with triclosan (TR)combined with ciprofloxacin on bacterial viability.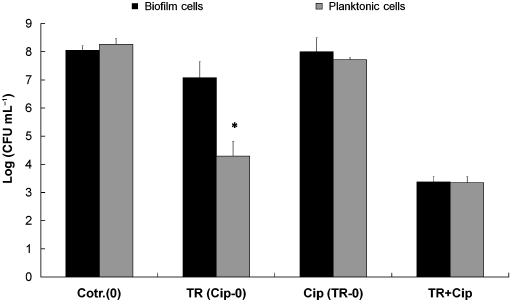 The effect of triclosan and ciprofloxacin in the biofilm
Fig. 4. Effect of treatment of SalmonellaTyphimurium with 500 mg mLÀ1 triclosan (TR) and500 mg mLÀ1ciprofloxacin (Cip) and combinedtreatments for 1 h on the viability of planktonic(gray) and biofilm-associated (black) cells. Dataare the average of three experiments; eachexperiment was conducted in duplicate.
The effect of triclosan and ciprofloxacin in the biofilm
Fig. 4. Effect of treatment of SalmonellaTyphimurium with 500 mg mLÀ1 triclosan (TR) and500 mg mLÀ1ciprofloxacin (Cip) and combinedtreatments for 1 h on the viability of planktonic(gray) and biofilm-associated (black) cells. Dataare the average of three experiments; eachexperiment was conducted in duplicate.